Abstract
The European LeukemiaNet (ELN) recently published the revised risk stratification system for AML in 2022 (ELN-2022). The risk stratification of ELN-2017 is generally focused on younger patients who are fit on standard induction treatment. Prognostic impact of the ELN risk stratification for elderly patients who treating low intensity chemotherapy is well not established. This retrospective cohort aimed to evaluate the prognostic significance of the revised ELN-2022 risk stratification in elderly patients treated decitabine chemotherapy. Total 123 patients with elderly AML were eligible.
Based on 2022 ELN risk stratification, 14 (11.4%) patients were classified as favorable, 53 (43.1%) as intermediate risk, and 56 (45.5%) as the high risk, respectively. The ELN-2022 classification re-assigned 27 (21.2%) of patients into more adverse-risk group, and 2 (2.4%) of patients into more favorable-risk group compared with the ELN-2017 recommendations. This difference was largely due to the FLT3-ITD allelic ratio which is no longer considered in the ELN 2022 risk classification. Moreover, myelodysplasia-related gene mutations including EZH2, SF3B1, SRSF2, STAG2, U2AF1 genes is now indicated as adverse risk which was another important change compared to previous ELN risk stratification. Because elderly patients highly associated with AML following prior MDS or MDS/MPN, the revised ELN-2022 is well presented an adverse prognosis in elderly AML patients.
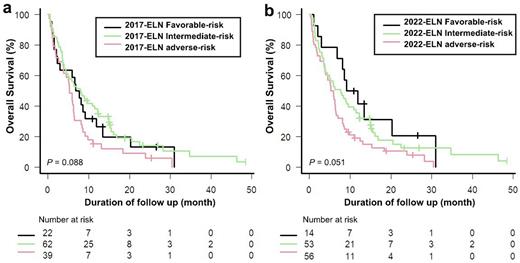
The rate of CR and CRi was 16.3% (20/123, CR = 8 patients, CRi =12 patients). There was no difference in overall response rate by ELN-2017 and ELN-2022 risk stratification. According to the ELN-2017 classification, the difference of 2-year overall survival (OS) between each risk group was not significant in 123 elderly patients treated decitabine (median OS of ELN-2017 favorable (n = 22), 7.2 months; intermediate-risk (n = 62), 8.0 months; Adverse-risk (n=39), 5.2 months; P = 0.081). In the revised 2022-ELN, the 2-year OS of each risk group was more distinct in elderly patients, compared to ELN-2017(median OS of ELN-2022 favorable (n = 14), 10.4 months; intermediate-risk (n = 53), 7.6 months; Adverse-risk (n=56), 5.5 months; P = 0.053).
The study presents the prognostic significance of revised 2022-ELN risk stratification in elderly AML patients receiving decitabine. This study suggests the possibility that the 2022-ELN classification could be predict survival in patients with AML for whom intensive induction therapy is not candidate.
Figure legendsFigure 1. Kaplan-Meier curves in patients with elderly AML according to (a) 2017-ELN (b) 2022-ELN.
Disclosures
Kim:Sanofi: Consultancy, Honoraria; Merck: Consultancy; Paladin: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; BMS: Research Funding; Novartis: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal